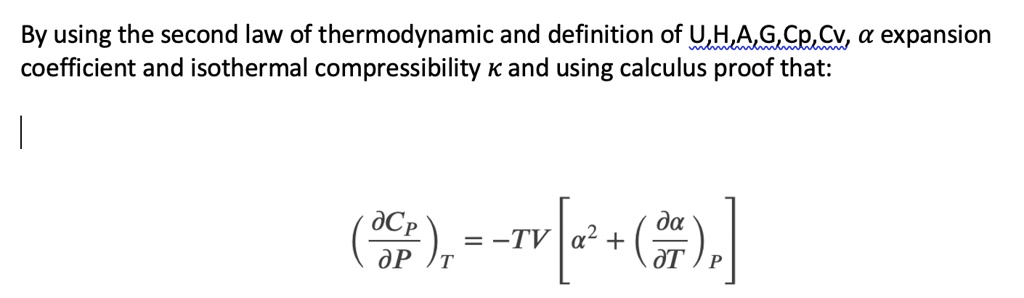
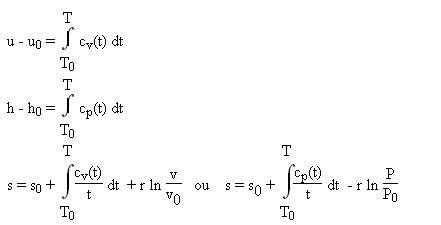Unit Four – First Law for Ideal Gases Outline Unit Four Goals Unit Four Goals Continued Unit Four Goals Continued Why Use Idea

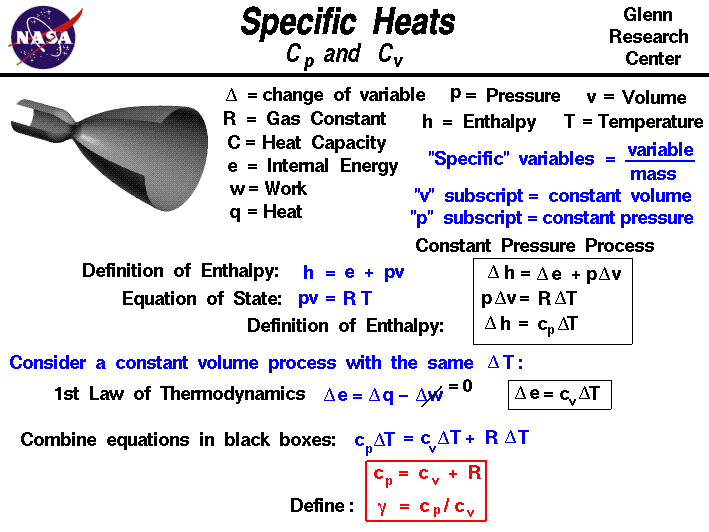
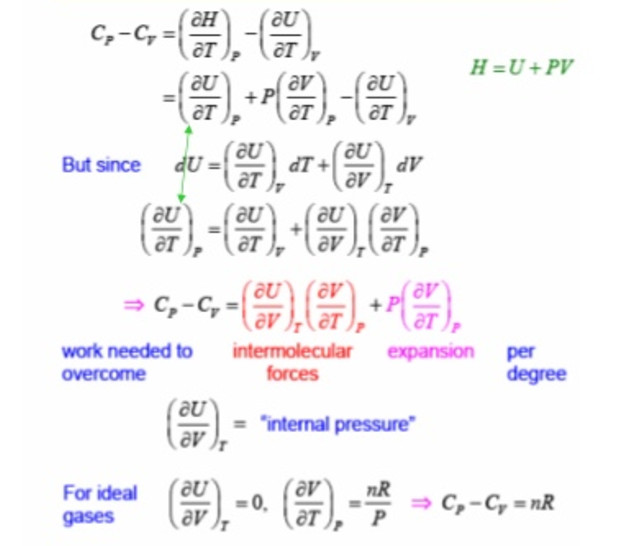
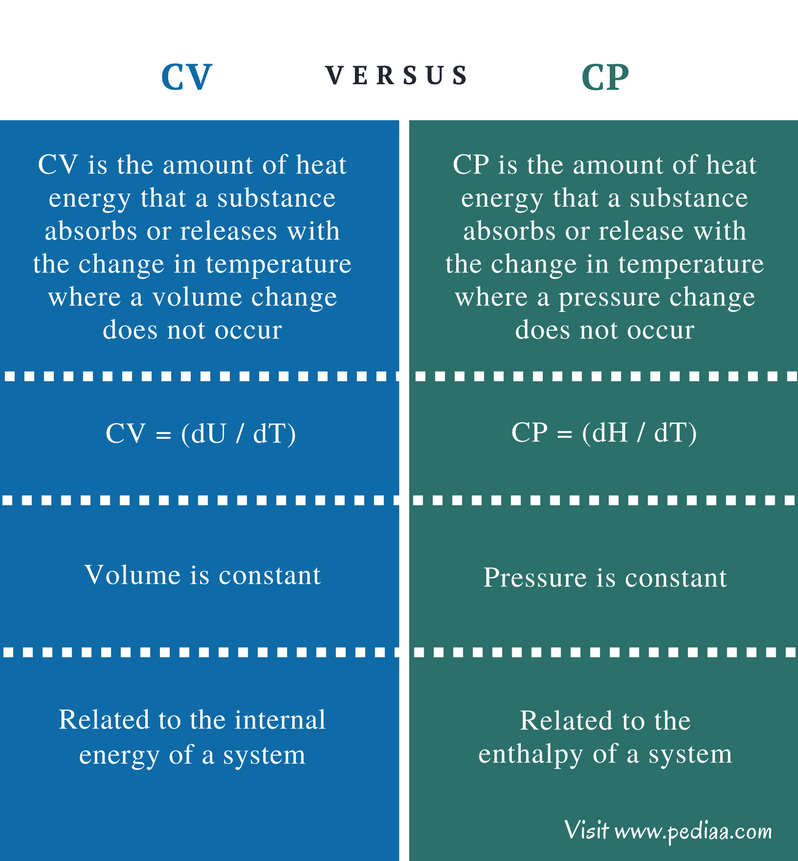
Relation Between Heat capacity at Constant Volume (CV) & Constant Pressure ( CP) - Chemistry - Aakash Byjus | AESL
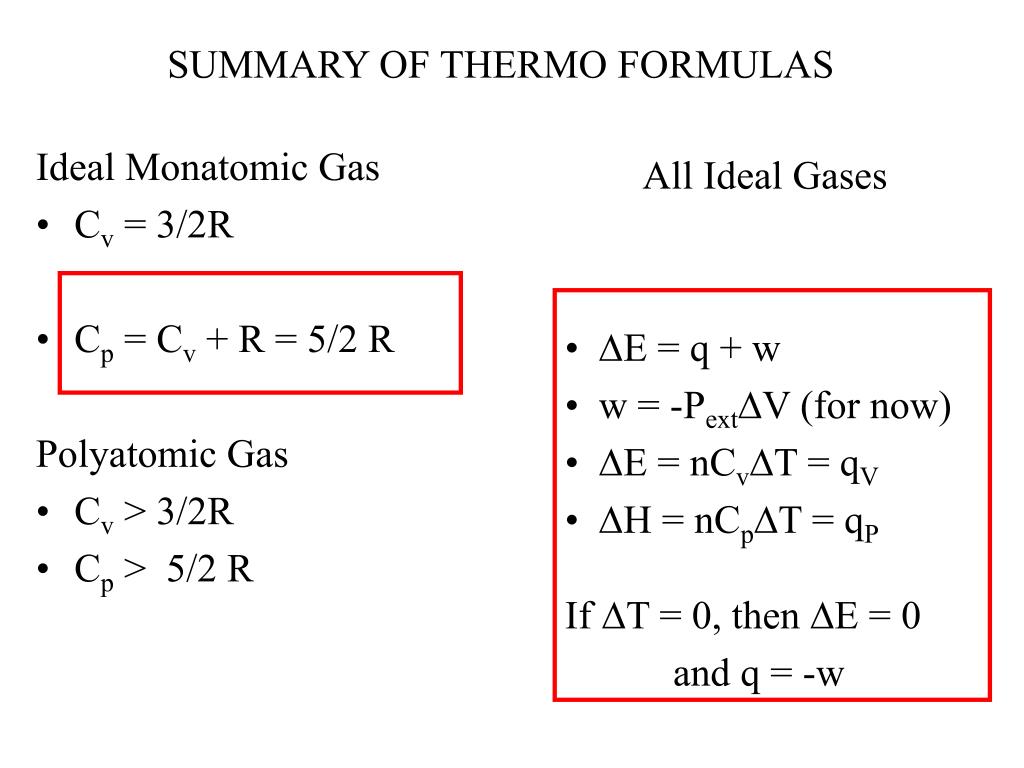
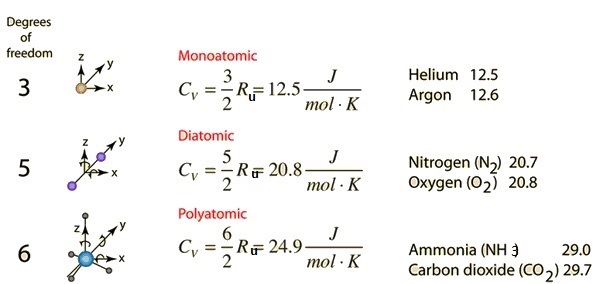
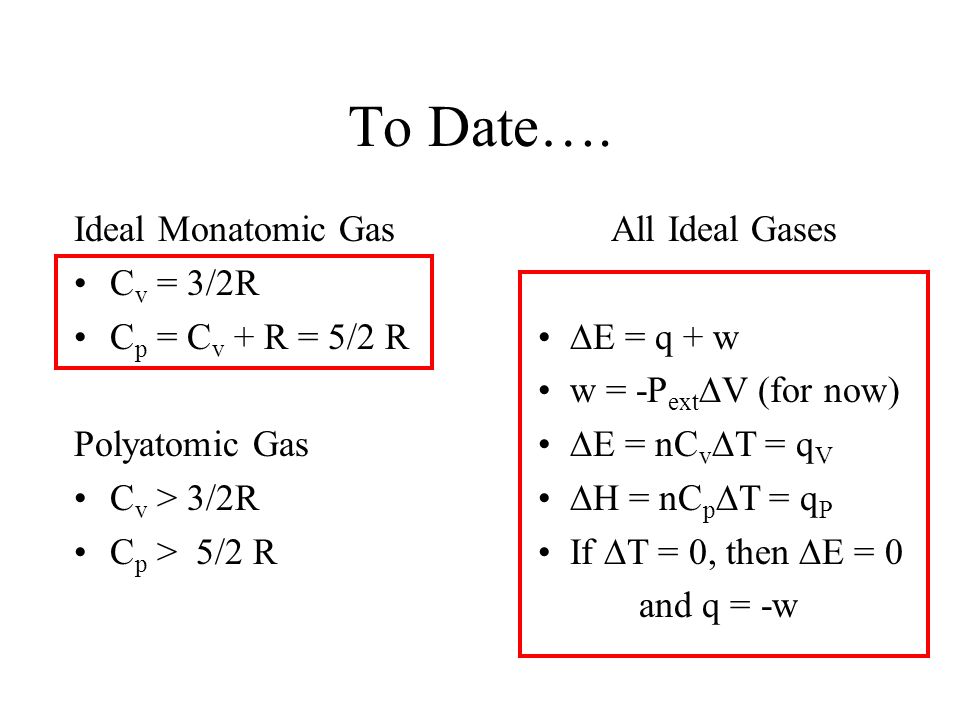
PPT - Ideal Monatomic Gas C v = 3/2R C p = C v + R = 5/2 R Polyatomic Gas C v > 3/2R C p > 5/2 R PowerPoint Presentation - ID:4355470
Use the thermodynamic relations to show that for an ideal gas CP − CV = R. - Sarthaks eConnect | Largest Online Education Community
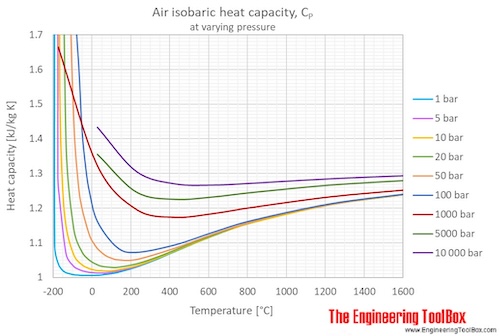
a Heat capacity ratio, C P /C V , as a function of temperature along... | Download Scientific Diagram

Cp-Cv for real gas, in terms of alpha & beta, change in internal energy with respect to volume - YouTube

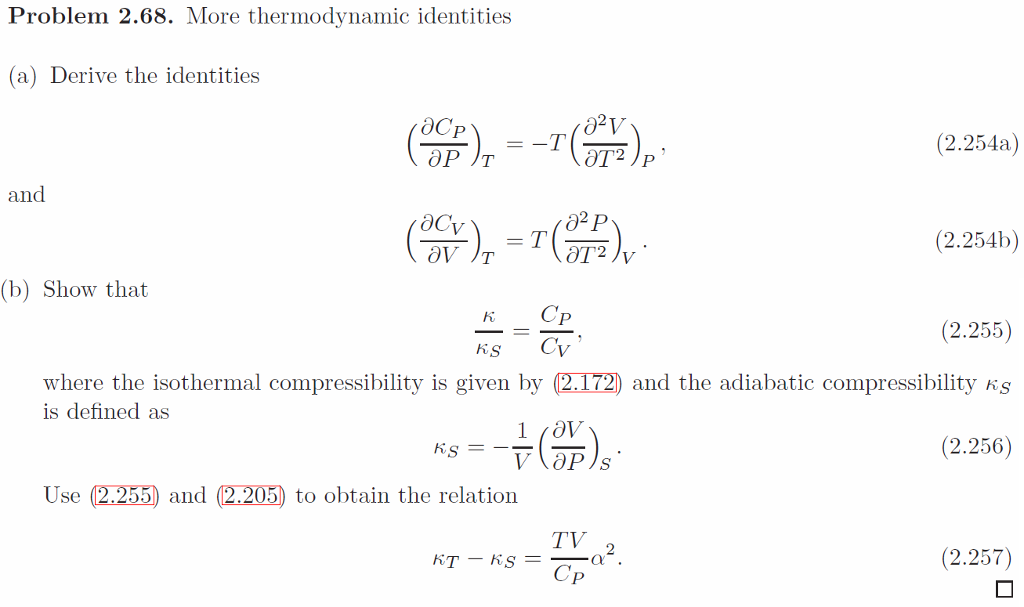
SOLVED: The Maxwell Relations in Q4 along with some clever manipulation can be used to derive general relationship between and Cp This questions walks through this process Using the thermodynamic identity; show