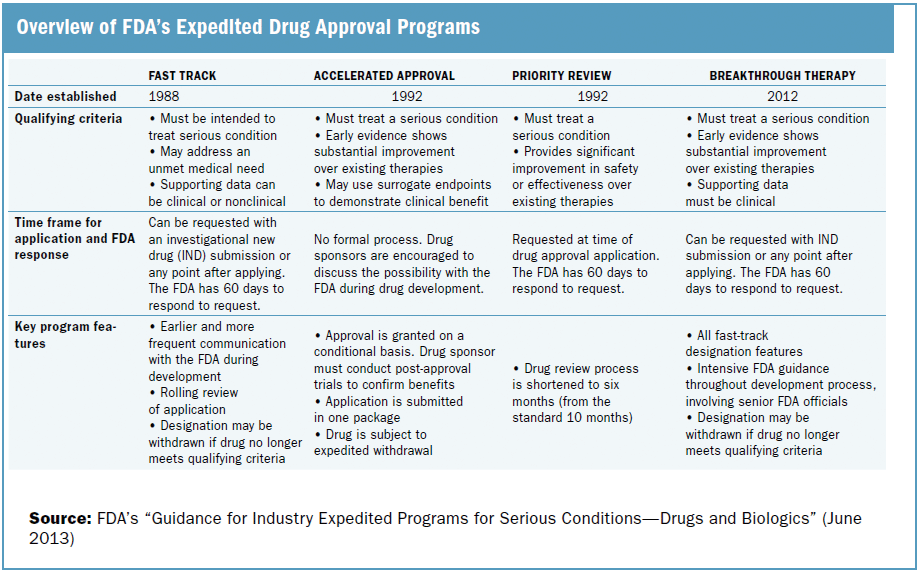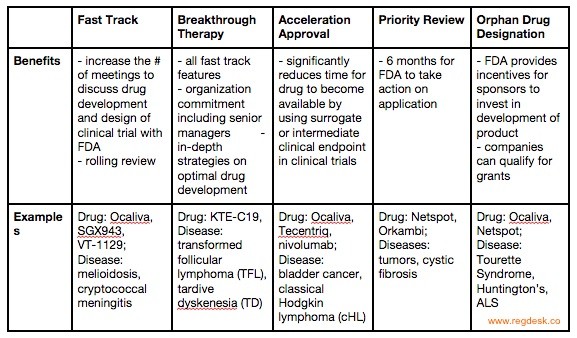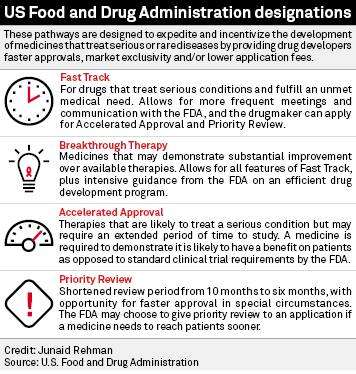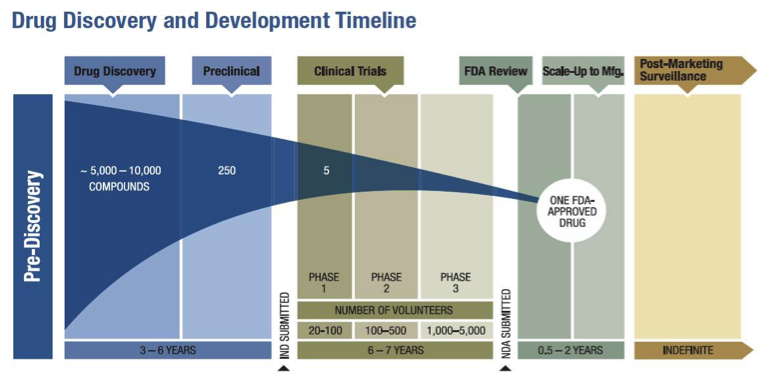
FDA efficiency for approval process of COVID-19 therapeutics | Infectious Agents and Cancer | Full Text

Regulatory Approval of Treatment for Ebola Virus: A U.S. and European Perspective - Science in the News
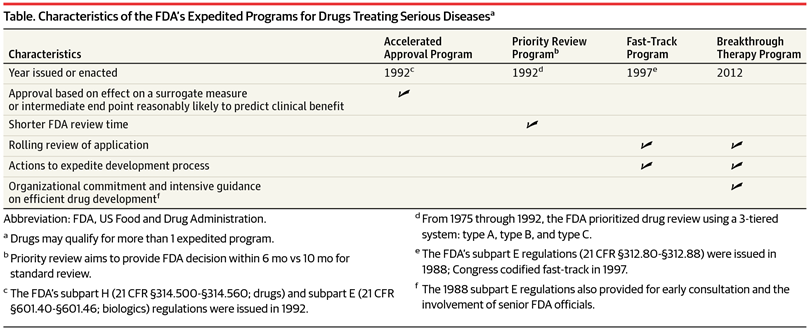
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB) | Seeking Alpha

FDA efficiency for approval process of COVID-19 therapeutics | Infectious Agents and Cancer | Full Text

OMEROS CORPORATION: FDA Confirms Omeros' Schedule for Rolling Review of the Company's BLA for Narsoplimab in the Treatment of HSCT-TMA | FDA Health News

Analysis of the Real-Time Oncology Review (RTOR) Pilot Program for Approvals of New Molecular Entities | SpringerLink

Update to Drugs, Devices, and the FDA: How Recent Legislative Changes Have Impacted Approval of New Therapies - ScienceDirect