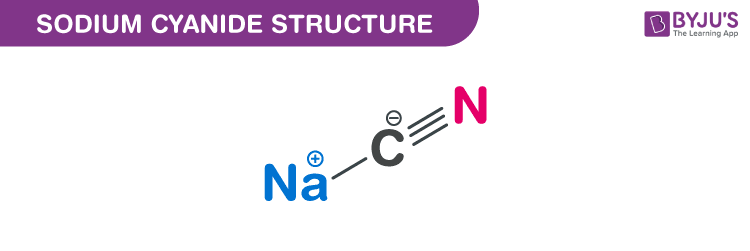
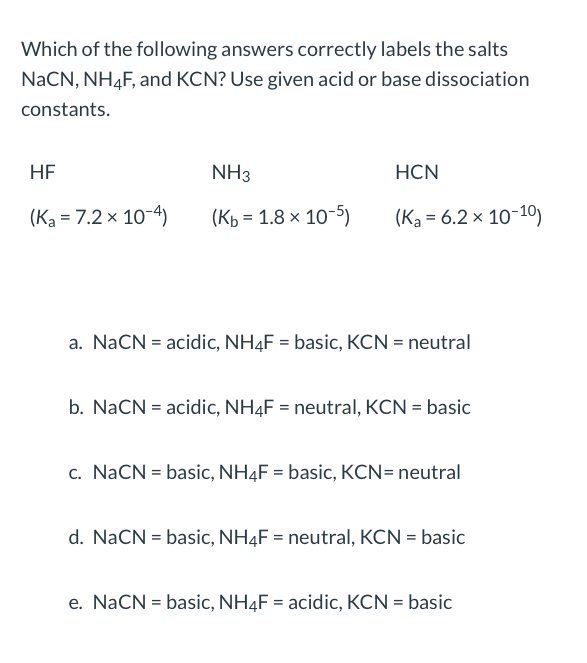
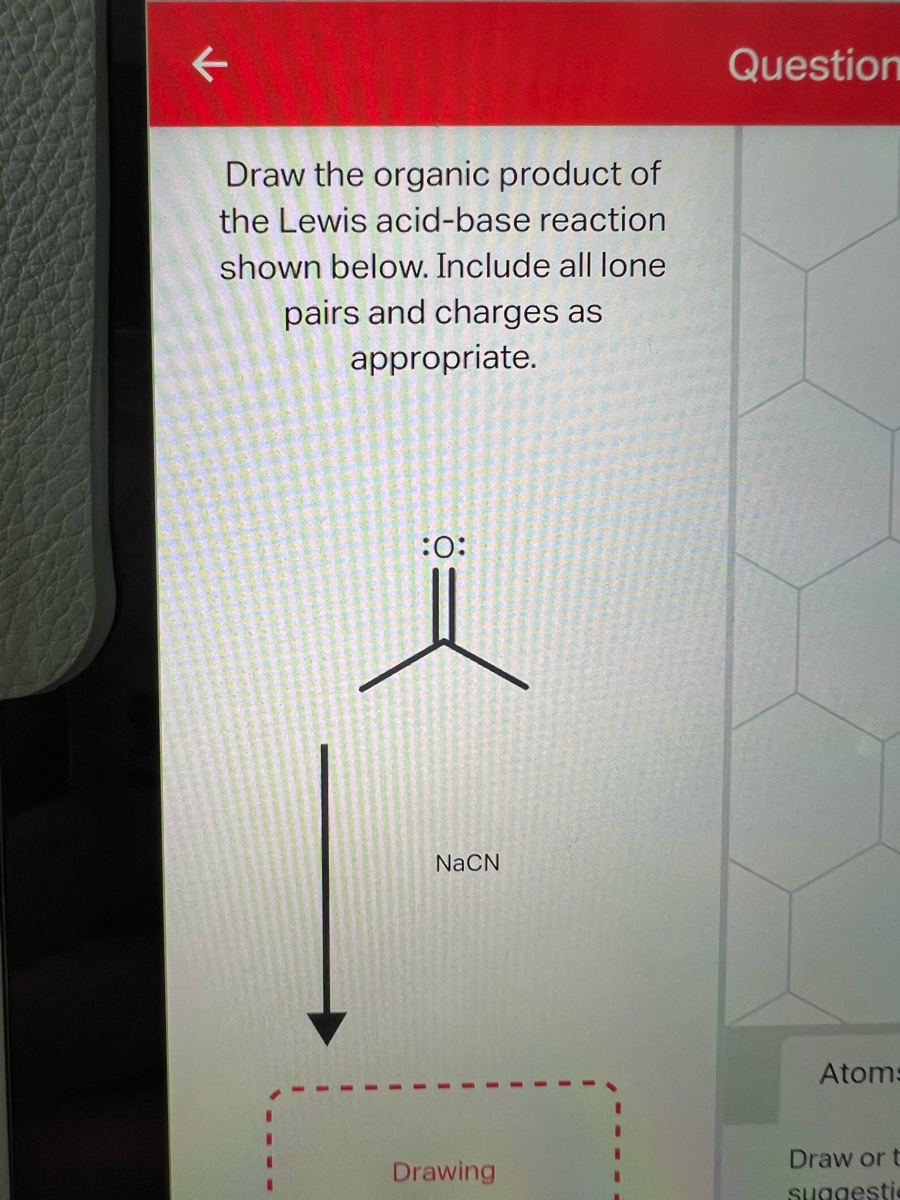
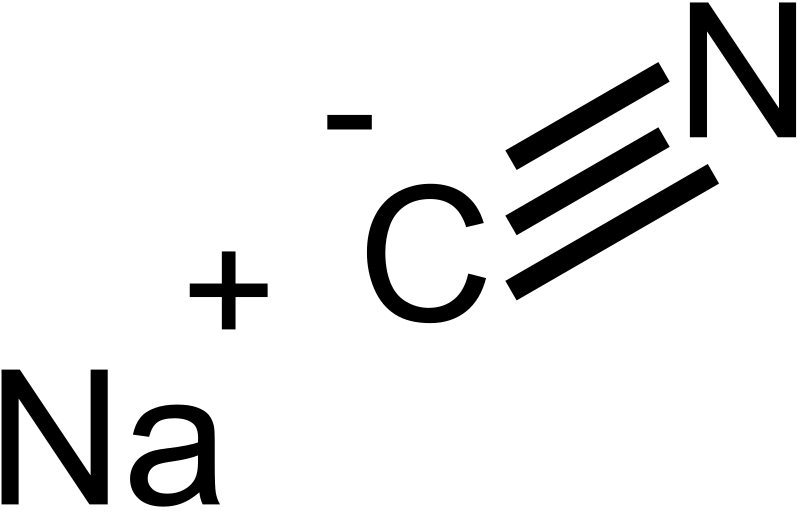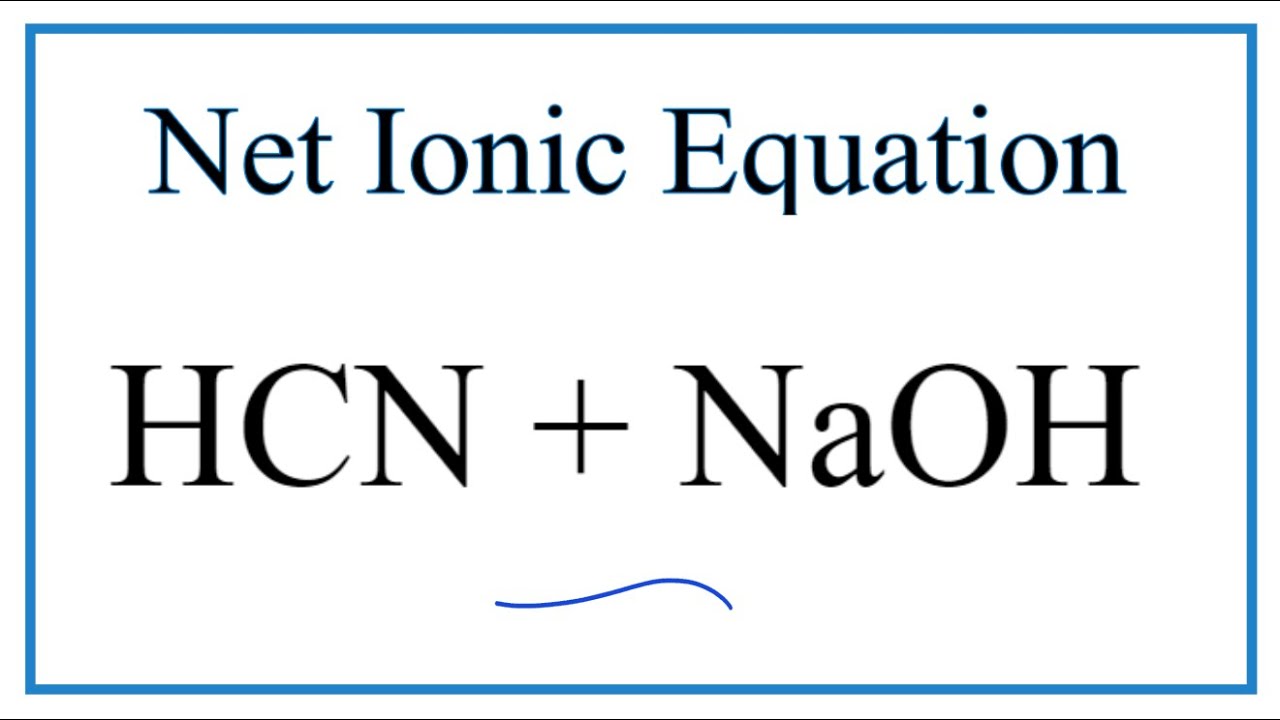acid base - Why do we need three equations to find the pH of NaCN, given Ka(HCN)? - Chemistry Stack Exchange

Which pair of compounds will form a buffer in aqueous solution? NaCN and KCN HCl and NaOH NaCN - Home Work Help - Learn CBSE Forum
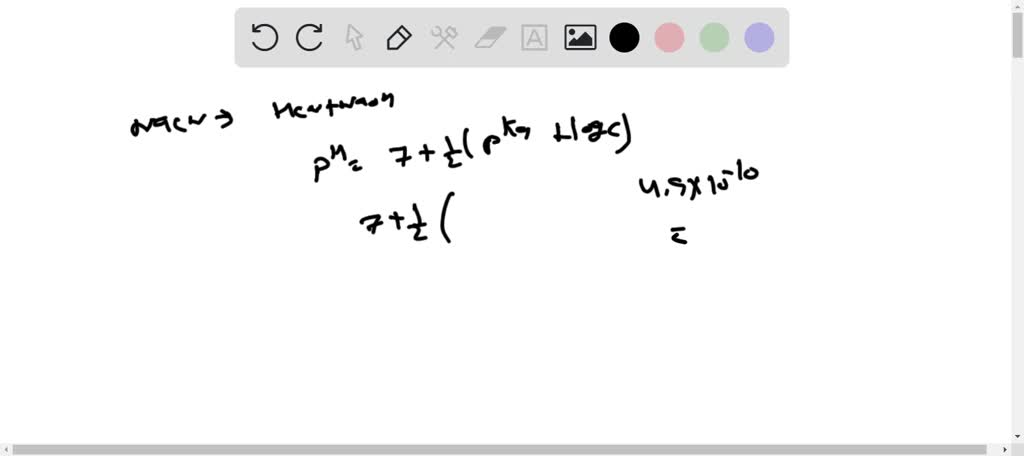
SOLVED: The weak acid hydrocyanic acid, HCN, and the strong base sodium hydroxide react to form the salt sodium cyanide, NaCN. Given that the value of Ka for hydrocyanic acid is 4.90×10−10,
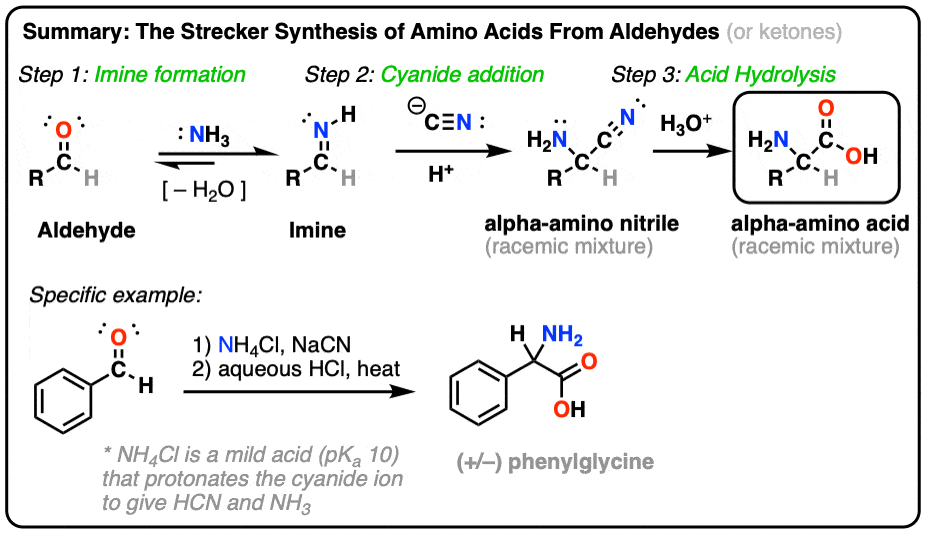
HYDROLYSIS OF SALTS Salt solutions may be acidic, basic, or neutral, depending on the original acid and base that formed the sal
✓ Solved: An unknown salt is either NaCN, NaC2H3O2, NaF, NaCl, or NaOCl. When 0.100 mole of the salt...

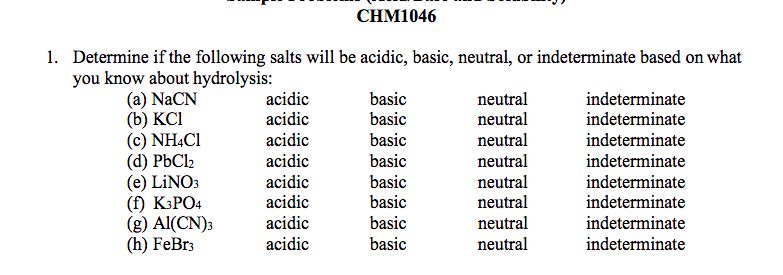
Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF